Supercapacitors |
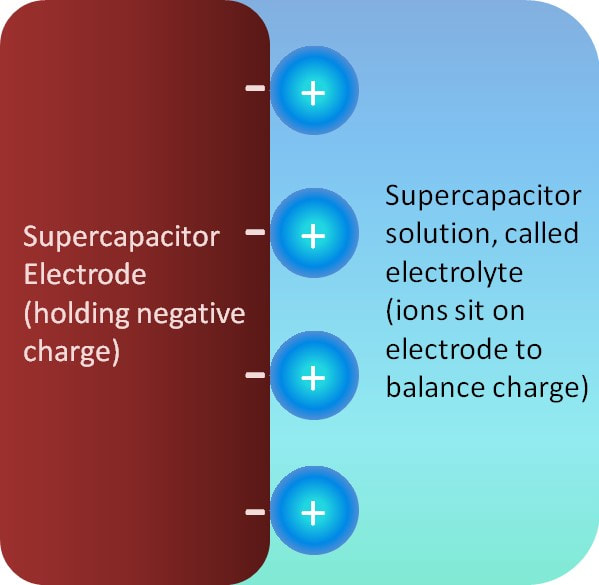
With rising oil prices and growing environmental awareness, people are becoming more interested in alternative energy systems (such as solar and wind power) and energy storage systems (batteries, fuel cells and supercapacitors). Our research focuses on supercapacitors, where charge is stored in very rapid reactions or on the surface of an electrode with a layer of oppositely-charged ions in solution (see figure). Supercapacitors have several benefits when compared to other energy storage systems. For instance, they work well for short power bursts (camera flashes or airbag deployment) and they can be charged many, many more times than typical batteries. Unfortunately, supercapacitors undergo a process called ‘self-discharge’, where the supercapacitor loses charge when it has been charged but not immediately used. For example, this could be a real problem if you go on vacation and leave your vehicle at the airport – by the time you return home, the supercapacitor may have lost all of its charge, and you would be unable to start your vehicle. Obviously, therefore, self-discharge may be a significant hindrance to the commercialization of supercapacitors. The present focus of the research in our lab is the identification of the causes of self-discharge. Ideally, if we can identify the causes (and mechanisms) of self-discharge we can find a way of minimizing/preventing self-discharge, making supercapacitors more commercially useful and viable. |
Biosensors |
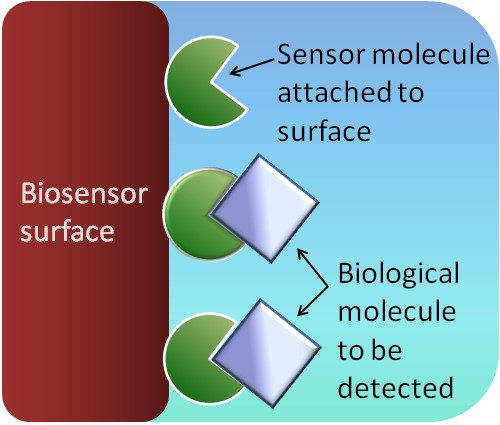
The types of biosensors that we study use a flow of electricity through a metal surface to quantify biologically important molecules attached to the surface (see figure). We are interested in the interactions between proteins and the surface. Ideally, these biosensors would be implanted directly into a person’s body to measure important biological molecules continuously and allow for an immediate response if the molecule of interest becomes too scarce or too abundant.
Using our knowledge of electrodes and interfaces we examine how proteins adsorb on surfaces, how they react with electrodes and how the electrode chemistry impacts the protein reactions. One important problem for biosensors is the difficulty in ensuring they quantify only the molecule of interest. Often a response maybe seen with multiple different molecules - this means that the biosensor indicates that the biologically relevant molecule is attached to the surface, when it is in fact an entirely different molecule. This is termed as an issue with selectivity. Selectivity is important since real biological systems contain many different proteins and other molecules and the biosensor needs to be able to selectively quantify only the desired molecule. Our research examines the parameters and methods that may improve selective quantification. If we can understand the important parameters, we may be able to devise a method to produce a more selective biosensor. |